-
PDF
- Split View
-
Views
-
Cite
Cite
Susanne Holck, Inger L. Holm, Peter P. Holck, Marianne Pedersen, Annette Nørgaard, Svend Norn, Henrik Permin, Leif P. Andersen, Epithelial cell kinetics of the gastric mucosa during Helicobacter pylori infection, FEMS Immunology & Medical Microbiology, Volume 50, Issue 2, July 2007, Pages 206–212, https://doi.org/10.1111/j.1574-695X.2007.00254.x
Close - Share Icon Share
Abstract
Helicobacter pylori is an important pathogen in major gastroduodenal diseases, including inflammation with ulceration and gastric malignancies. Alterations in H. pylori associated cell turnover in gastric epithelial cells are examined in relation to inflammatory activity, bacteria load and cytokines which may improve knowledge concerning the outcome of gastric diseases caused by H. pylori. Antral biopsies from 42 dyspeptic patients including 27 H. pylori-positive and 15 H. pylori-negative patients were tested for apoptotic activity by the TUNEL assay, and immuno-histochemically for p53 and the proliferative marker Ki-67. H. pylori infection, bacteria load and inflammatory activity were associated with increased cell turnover as judged by enhanced activities of TUNEL, p53 and Ki-67. Only p53 was significantly correlated to IFN-γ, IL-8 and IL-10. The H. pylori-positive state was furthermore accompanied by varying degrees of altered distribution pattern of the markers studied, with occasional presence of apoptosis in the deeper pit zones, upward extension of Ki-67 and to a lesser degree of p53. Given a similar pattern of change in proliferation and apoptosis in some neoplastic lesions in other parts of the gastrointestinal tract, such studies in cell turnover may provide insights valuable in the investigations of potential precursors of gastric malignancies.
Introduction
Helicobacter pylori is an important pathogenic factor in chronic gastritis, duodenal and gastric ulcers, and it increases the risk of development of gastric malignancies (Graham, 1991; Korman, 1993; Kreiss, 1995; Peek & Blaser, 2002). Helicobacter pylori colonizes the gastric mucosa, initiating alterations in the normal balanced cell turnover in gastric epithelial cells by changing the activity of apoptosis and/or cell proliferation, which ultimately may lead to ulceration or cancer. Various factors are involved in the regulation of the balance between apoptosis and proliferation, such as CD95 (Fas), p53, the bcl-2 family and caspases (Rudi, 1998; Konturek, 1999; Wagner & Beil, 2001; Xia & Talley, 2001; Hofseth, 2004). Upregulation during H. pylori infection of IFN-γ, IL-8 and other cytokines may contribute to enhanced apoptosis (Lindholm, 1998; Lehmann, 2002).
Several investigators have reported enhancement of cell proliferation and apoptosis in the gastric epithelium during H. pylori infection (Brenes, 1993; Jones, 1997; Jang & Kim, 2000). Others fail, however, to establish such a straightforward correlation (Peek, 1997; Rokkas, 1999; Cover, 2003; Freitas, 2004), emphasizing the importance of diversities of the strains. Additionally, divergent reports may, at least in part, reflect variations in study populations and in the choice of study design.
To increase understanding of the pathogenesis of the H. pylori infection, regulation of the cell proliferation and cell death are, however, important issues to pursue. In the present study the gastric epithelial cell kinetics were examined by the TUNEL method, which visualizes apoptotic bodies and immunohistochemically for the nuclear regulatory protein p53 and the proliferation marker Ki-67. Assessment of extent and distribution of these variables were correlated to the inflammatory activity and bacteria density in gastric mucosa from H. pylori-positive and H. pylori-negative patients.
Materials and methods
Patients
This analysis is based on material from a previous study, in which H. pylori status, density of bacteria, and immunoexpression of some interleukins were examined (Holck, 2003). The present analysis is, however, confined to those biopsies which are optimally oriented, (n=42, 21 males, 21 females). Helicobacter pylori status determined by culture and histology (Holck, 2003) showed that 27 patients were H. pylori-positive, 15 were H. pylori-negative. Among the H. pylori-infected patients, ulcers were gastroscopically demonstrated in 11 patients (two gastric, three prepyloric, six duodenal), whereas none of the H. pylori-negative patients had developed ulcers. Histologically, all H. pylori-positive biopsies featured a chronic active gastritis, as well as intestinal metaplasia in seven of these cases. There was no correlation between the presence of ulcer and intestinal metaplasia. The group of H. pylori-negative individuals had normal mucosa in six cases, whereas nine cases featured an inactive chronic gastritis. Metaplasia was not apparent in these cases.
Procedure for Ki-67 and p53
Formalin-fixed paraffin-embedded sections of antral gastric biopsies were rehydrated. Endogenous peroxidase was inhibited in the sections by exposure to 3% hydrogen peroxide for 10 min, followed by washing in running tapwater and TBS (Tris-buffered saline, pH 7.6). The sections were microwave pretreated at 100°C for 15 min in Tris-EGTA buffer, pH 9, cooled to room temperature and washed in TBS. Subsequently, the sections were incubated at room temperature for 30 min with antibodies to Ki67 (M7240, 1:250, MIB1) and p53 (M7001, 1:1000, DO-7), diluted in TBS containing 1% bovine serum albumin. The sections were washed in TBS, treated with En Vision (K5007, DakoCytomation, DK A/S) at room temperature for 30 min, washed with TBS and exposed to DAB, diluted 1:50 in the kit buffer for 10 min at room temperature. The immunostaining reaction was blocked by tapwater and the sections were counterstained with hematoxylin. Brown nuclear staining indicated positive immunohistochemical reaction. Adenocarcinoma tissues from the colon were used as positive controls. Replacement of the primary antibodies with TBS was used for the negative controls.
Procedure for TUNEL assay
Formalin-fixed paraffin-embedded sections of gastric antral biopsies were digested with proteinase K (Roche cat. no. 3115879) 0.2 mg mL−1 PBS for 5 min at 37°C. After washing in water, the sections were exposed to hydrogen peroxide as described above, and were washed again in water and PBS. Apoptosis was detected by the TUNEL technology (In situ cell death detection kit, POD, Roche cat. no. 1684817), according to the guidelines of the manufacture. In brief, sections were treated with TUNEL reaction mixture at 37°C for 1 h in dark and humid conditions, followed by exposure to the converter-POD at 37°C for 30 min. Immunostaining was revealed as described above. Lymph nodes were used as positive controls and for negative controls the transferase was omitted.
Scoring and quantification
All immunostained sections were randomized and blinded for clinical/endoscopic finding as well as for previous scores of inflammation, H. pylori status and density, and for interleukins. The morphological evaluation was primarily performed by two senior morphologists with diverse expert status [in-depth knowledge regarding histochemical and immunohistochemical aspects of normal tissues (IH) and with particular interest in gastrointestinal pathology (SH), respectively]. After a period of independent studies, all sections were jointly reviewed and discussed. Subsequently, one of the authors (IH) recorded the percentage of positive epithelial cells: (1) regardless of the microanatomic distribution and (2) in the following compartments: surface and superficial third of foveolae (f1), middle third of foveolae (f2), deep third of foveolae and isthmus (f3), using four-tiered scales (0, 0%; 1, <1%; 2, 1–10%; and 3, >10% for epithelial cells positive for TUNEL assay and for p53 and 0, 0%; 1, <10%; 2, 10–50%; and 3, >50% for epithelial cells positive for Ki-67). Compartments were evaluated in five randomly chosen gastric pit zones.
Statistical analysis
Fisher's exact test was used to verify a significant association between the parameters. A P value <0.05 was considered statistically significant.
Results
Scorings regardless of microanatomic distribution
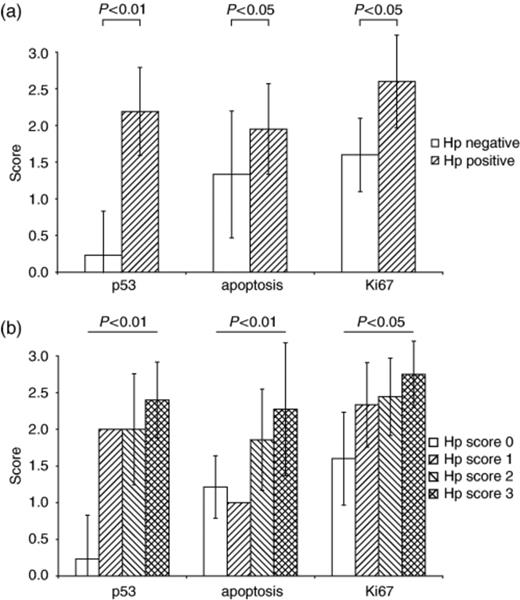
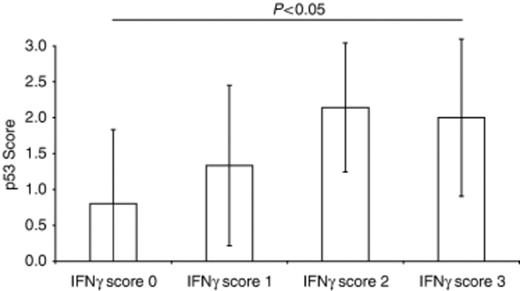
Evaluation of the entire area of the antral sections from H. pylori-positive and H. pylori-negative patients showed that H. pylori status as well as the inflammatory activity (Holck, 2003) were significantly correlated with the following parameters: TUNEL-positive epithelial cells (P<0.05), p53-positive cells (P<0.01) and Ki-67-positive cells (P<0.05) (Fig. 1a). Significant associations were also obtained between H. pylori density, detailed in a previous communication (Holck, 2003) and the scores of TUNEL-positive cells (P<0.01), p53 (P<0.01) and Ki-67 (P<0.05) (Fig. 1b). The expression of the cytokines IFN-γ, IL-8 and IL-10, previously scored in these biopsies (Holck, 2003), correlated with p53 (P<0.05) (Fig. 2), but not with TUNEL and Ki-67. None of the markers studied correlated with the presence or absence of ulcers, nor with the presence or absence of intestinal metaplasia.
Scores of p53, TUNEL (apoptotic bodies), and Ki-67 as a function of H. pylori status (a) and as a function of H. pylori load, as previously detailed (Holck, 2003) (b). The results are mean±SD. Scores of the three markers as a function of inflammatory activity (Holck, 2003) were similar to that demonstrated in (a) (not shown).
Scores of p53 as a function of the scores of the pro-inflammatory interleukin IFN-γ, previously detailed (Holck, 2003). The results are mean±SD. Similar results were obtained for IL-8 and IL-10 (not shown).
Scorings of compartmental distribution
The topographic profiles of the labelled cells along the gastric pits appear in Table 1 and Fig. 3. It can be seen that the most consistent pattern observed among the H. pylori-negative cases was a scarce apoptosis (TUNEL positive) confined to superficial zones and proliferation (Ki67 positive) involving the deeper portions of the gastric pits. Traces of p53-expression were occasionally discerned in the deep portions of the pits.
Average values of scores of TUNEL, Ki-67 and p53 in relation to the level of the compartments recorded for the four histologically defined subgroups
| Tunel | Ki-67 | P53 | ||||||||||
| Compartments | N | ICG | CAG1 | CAG2 | N | ICG | CAG1 | CAG2 | N | ICG | CAG1 | CAG2 |
| n | 6 | 9 | 15 | 12 | 6 | 9 | 15 | 12 | 6 | 9 | 15 | 12 |
| f1 | 0 | 0 | 2 | 2 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| f2 | 0 | 0 | 0 | 3 | 2 | 1 | 2 | 2 | 0 | 0 | 1 | 1 |
| f3 | 0 | 0 | 0 | 1 | 2 | 2 | 3 | 3 | 0 | 0 | 2 | 2 |
| Tunel | Ki-67 | P53 | ||||||||||
| Compartments | N | ICG | CAG1 | CAG2 | N | ICG | CAG1 | CAG2 | N | ICG | CAG1 | CAG2 |
| n | 6 | 9 | 15 | 12 | 6 | 9 | 15 | 12 | 6 | 9 | 15 | 12 |
| f1 | 0 | 0 | 2 | 2 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| f2 | 0 | 0 | 0 | 3 | 2 | 1 | 2 | 2 | 0 | 0 | 1 | 1 |
| f3 | 0 | 0 | 0 | 1 | 2 | 2 | 3 | 3 | 0 | 0 | 2 | 2 |
Compartments: surface/superficial third of foveolae (f1), middle third of foveolae (f2), deep third of foveolae/isthmus (f3).
N, normal mucosa (Hp−, i.e. H. pylori-negative cases); ICG, inactive chronic gastritis (Hp−); CAG1, chronic active gastritis with no deep apoptosis (Hp+); CAG2, chronic active gastritis with deep apoptosis (Hp+).
Scores: 0, 0%+ epithelial cells or merely traces of expression; 1, <1%+ epithelial cells+ for TUNEL assay and p53, <10% epithelial cells+ for Ki-67; 2, 1–10% epithelial cells+ for TUNEL assay and p53, 10–50% epithelial cells+ for Ki-67; 3, >10% epithelial cells+ for TUNEL assay and p53, >50% epithelial cells+ for ki-67.
Average values of scores of TUNEL, Ki-67 and p53 in relation to the level of the compartments recorded for the four histologically defined subgroups
| Tunel | Ki-67 | P53 | ||||||||||
| Compartments | N | ICG | CAG1 | CAG2 | N | ICG | CAG1 | CAG2 | N | ICG | CAG1 | CAG2 |
| n | 6 | 9 | 15 | 12 | 6 | 9 | 15 | 12 | 6 | 9 | 15 | 12 |
| f1 | 0 | 0 | 2 | 2 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| f2 | 0 | 0 | 0 | 3 | 2 | 1 | 2 | 2 | 0 | 0 | 1 | 1 |
| f3 | 0 | 0 | 0 | 1 | 2 | 2 | 3 | 3 | 0 | 0 | 2 | 2 |
| Tunel | Ki-67 | P53 | ||||||||||
| Compartments | N | ICG | CAG1 | CAG2 | N | ICG | CAG1 | CAG2 | N | ICG | CAG1 | CAG2 |
| n | 6 | 9 | 15 | 12 | 6 | 9 | 15 | 12 | 6 | 9 | 15 | 12 |
| f1 | 0 | 0 | 2 | 2 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| f2 | 0 | 0 | 0 | 3 | 2 | 1 | 2 | 2 | 0 | 0 | 1 | 1 |
| f3 | 0 | 0 | 0 | 1 | 2 | 2 | 3 | 3 | 0 | 0 | 2 | 2 |
Compartments: surface/superficial third of foveolae (f1), middle third of foveolae (f2), deep third of foveolae/isthmus (f3).
N, normal mucosa (Hp−, i.e. H. pylori-negative cases); ICG, inactive chronic gastritis (Hp−); CAG1, chronic active gastritis with no deep apoptosis (Hp+); CAG2, chronic active gastritis with deep apoptosis (Hp+).
Scores: 0, 0%+ epithelial cells or merely traces of expression; 1, <1%+ epithelial cells+ for TUNEL assay and p53, <10% epithelial cells+ for Ki-67; 2, 1–10% epithelial cells+ for TUNEL assay and p53, 10–50% epithelial cells+ for Ki-67; 3, >10% epithelial cells+ for TUNEL assay and p53, >50% epithelial cells+ for ki-67.
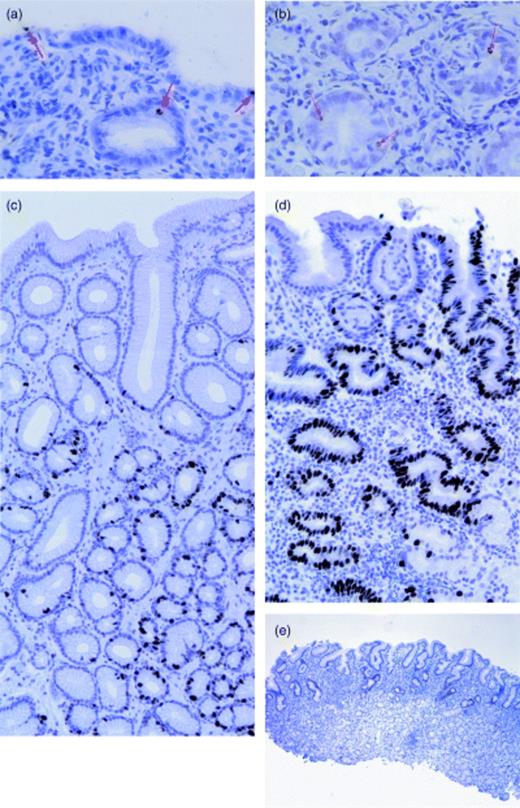
Apoptotic activity, visualized by the TUNEL method in a H. pylori-positive specimen in the superficial compartment (a) and in the deep compartment (b). Apoptotic bodies were scarce in H. pylori-negative specimens (not shown). Immunohistochemical expression of nuclear Ki-67, confined to the deep compartment in a H. pylori-negative biopsy, visualizing the normal regenerative zone, as opposed to the nonreaction of the more superficial cells (c), expanding of the Ki-67-positive lining cells to the superficial compartments in a H. pylori-positive specimen (d). Note the tortuous course of the foveolae lined by extensively labelled epithelial cells, as opposed to the straight course of the more sparsely decorated specimens. Immunostaining of p53 is readily apparent in H. pylori-positive specimens (e), as opposed to its virtual inapparence in H. pylori-negative biopsies (not shown).
In addition to a generalized up-regulation of the three cell turnover markers, the H. pylori infection was in some cases accompanied by an altered distribution pattern, particularly of the apoptosis, to a lesser extent of Ki-67. Thus, two different profiles of apoptosis were observed within this group of patients, featuring apoptosis restricted to the superficial zones and apoptosis expanding to the deeper pits, respectively. The former characterized 15 biopsies (five males, 10 females), seven of which comprising focal intestinal metaplasia. The remaining 12 biopsies (eight males, four females), which featured extended apoptosis, were not intestinalized. An expansion with aberrant location also characterized the Ki-67 staining, which in some specimens resided in the superficial portion of the mucosa. The p53-positive nuclei appeared in the deeper pit zones with occasional expansion in the superficial zone.
Discussion
Correlation between extent of cell turnover markers and histology
This investigation of gastric epithelial cell turnover in dyspeptic patients showed that H. pylori status and load as well as inflammatory activity were associated with enhanced apoptosis and proliferation of epithelial cells visualized by the TUNEL method and immunohistochemically for Ki67 and p53, corroborating several previous studies, in children and adults, including diverse clinical settings (gastritis as well as gastric ulcer and carcinoma) (Brenes, 1993; Jones, 1997; Jang & Kim, 2000). The role exerted by the virulence factors CagA and VacA on these dynamic processes has previously been addressed; however, the available results are conflicting (Peek, 1997; Wagner, 1997; Rokkas, 1999; Galmiche, 2000; Moss, 2001; Cover, 2003; Freitas, 2004). The VacA and CagA status was not determined in the present material.
Topographic distribution pattern of cell turnover markers
The distribution pattern of the cell turnover markers here studied is largely in concert with results of other topographic analyses (Moss, 1996; Jones, 1997; Jang & Kim 2000; Lehmann, 2002; Sougioultzis, 2003). Of note is the occasional observation of apoptotic activity of the deeper compartments of the H. pylori-positive mucosa. Such an aberrant location of apoptosis has previously been found in the intestinalized mucosa (Xia & Talley, 2001). The present study could not, however, reproduce this correlation, apoptosis being restricted to the superficial zone in our seven biopsies featuring intestinal metaplasia. The subtype of intestinal metaplasia, determined by its histochemical constituents of the mucin, as well as the topographic location and extent of the intestinal metaplasia may all play a role. The overexpression and aberrant distribution of p53 and ki-67 to more superficial layers in H. pylori-positive biopsies likewise conform to the observations of other investigators (Jones, 1997; Jang & Kim, 2000). Thus, H. pylori infection is associated with an enhanced expression and extension of the proliferative area from deeper to more superficial zones. How the distribution pattern of these events is influenced by CagA and VacA status is unclear. Studies of this issue may however further advance our understanding of the mechanisms involved in H. pylori-associated changes of the gastric mucosa.
Correlation between cell turnover markers and interleukins
In a previous study it was shown that H. pylori infection, as well as bacterial load and inflammatory activity, were associated with the expression of IL-8, IL-10 and IFN-γ (Holck, 2003). The present observation of a significant correlation between the expression of p53 and IL-8, IL-10 and IFN-γ is intriguing. Whether this correlation is based on a causal relation between p53 and the cytokines is unclear. It is, however, relevant to recall the proposed speculation, that neutrophils activated by IL-8 synthesize reactive oxygen-radicals in patients infected with H. pylori which could cause mutations in gastric epithelial cells and lead to p53 mutation (Nguyen, 1992; Zhang, 1997; Morgan, 2003; Taguchi, 2005). This hypothesis might explain the correlation recorded between IL-8 and immunoexpression of p53. Few investigations are available on the relation between interleukins and cell turnover markers. In one study a significant correlation was observed between apoptosis and some interleukins, including IFN-γ (Lehmann, 2002). In an experimental study, no significant correlation was demonstrated between IFN-γ transcript levels and apoptosis or proliferation (Crabtree, 2004). Additional studies on the influence of these cytokines on the outcome of the H. pylori infection are needed.
Cell turnover markers in relation to predysplasia
Despite the well-established association between mutant p53 and neoplasia, the mere presence of p53 nuclear protein, apparent in the majority of our H. pylori-positive cases should not be construed as evidence of preneoplasia, nor can the absence of p53 expression exclude the subsequent development of dysplasia (Soong, 1996). Indeed, our results of up-regulated p53 activity demonstrated using the antibody DO-7 in H. pylori-positive biopsies may indicate an overexpression of wild-type p53 or the presence of mutant protein. A cohort of variables other than p53 aspects, including genomic polymorphism of the host, virulence of H. pylori, as well as diverse environmental factors, may influence the final outcome. Thus, based on studies at the protein level our possibilities for identifying predysplastic changes are rather limited. On the other hand, if attention is paid to the distribution pattern of the markers, as done in the present study, rather than only to their presence, the value of information may be augmented. Thus, the present observations on the imbalance of proliferation and apoptosis, which characterized a subgroup of our H. pylori-positive cases, mimic the results of similar cell kinetic studies on premalignant and malignant changes in other parts of the gastrointestinal tract, which have been the subject of extensive investigations (Mikami, 2003; Boman, 2004; Lörinc, 2005). Most interestingly, dysregulation of histologically normal intestinal mucosa in patients prone to develop carcinoma, such as familial adenomatous polyposis patients, has been recorded (Biasco, 2004). Such knowledge may be utilized in the pursuit of potential precursors of carcinoma in histologically unremarkable gastric mucosa. Though the population in this study is small, it is furthermore noteworthy that eight of 12 H. pylori-positive biopsies featuring deeply located apoptosis were derived from males, as opposed to a female predominance (10 of 14) among the H. pylori-positive biopsies with apoptotic activity confined to the superficial portion of the mucosa. Our observations corroborate the well-established knowledge of an even gender distribution of H. pylori-infection, as opposed to a male predominance among individuals, whose H. pylori-related gastric lesions are prone to morphologic progression. The malignant potential of a persistent imbalance between proliferation and apoptosis of the mucosa of the gastrointestinal tract has previously been suggested (Fox & Wang, 2001; Mc Namara, 2003). It remains however, to be seen whether deeply sited apoptosis play a particular role in gastric carcinogenesis. Though the results should be interpreted with caution, the hypothesis that an altered distribution of apoptosis may signify an early predysplastic event is intriguing.
In summary, we found that H. pylori infection was associated with an increase in proliferation and apoptosis. Additionally, the distribution pattern of these parameters displays some diversity with occurrence of dysregulation in some cases leading to altered cell kinetics. The latter may be of particular note, since it may create an environment conducive to the development of premalignant changes in some individuals, similar to observations in other parts of the gastrointestinal canal.
Acknowledgements
This study was supported by the Region 3 Fond, Denmark. The excellent technical assistance of Lis Christiansen is gratefully acknowledged.
References